 Home
Home
 Back
Back
Definition: This calculator computes the root mean square (RMS) velocity (\(v_{rms}\)), the average speed of gas particles in an ideal gas, based on temperature and molar mass.
Purpose: It is used in thermodynamics, statistical mechanics, and chemistry to analyze gas particle motion, aiding in understanding gas behavior, diffusion, and pressure.
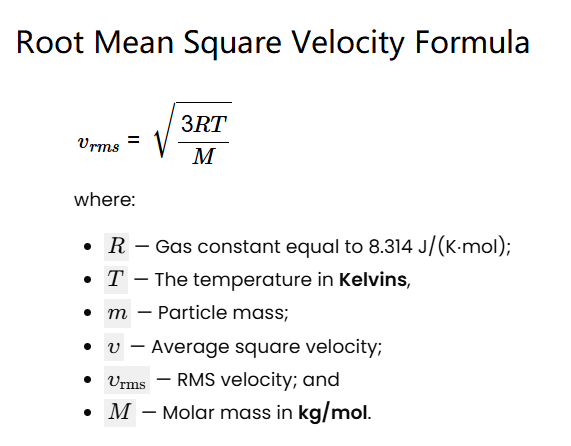
The calculator uses this formula:
Explanation: Select a gas or enter temperature and molar mass in your chosen units. The calculator converts to base units (K, kg/mol) and outputs RMS velocity in m/s, km/s, mph, ft/s, and Hz.
Unit Conversions:
Details: RMS velocity is key to gas kinetics and thermodynamics. Examples include:
Applications: Essential for gas laws, atmospheric science, and chemical engineering.
Tips: Select a gas to auto-fill molar mass or enter custom values with positive values and up to 4 decimal places. Results are in m/s, km/s, mph, ft/s, and Hz. Values < 0.0001 use scientific notation. Avoid zero temperature or molar mass.
Example: For oxygen gas (\(M = 32 \, \text{g/mol} = 0.032 \, \text{kg/mol}\), \(T = 293 \, \text{K}\)):
Average Velocity: \(v_{avg} = \sqrt{\frac{8RT}{\pi M}}\), slightly less than \(v_{rms}\).
Most Probable Velocity: \(v_{mp} = \sqrt{\frac{2RT}{M}}\), the peak of the Maxwell-Boltzmann distribution.
Kinetic Theory of Gases: Relates \(v_{rms}\) to pressure, temperature, and density via \( P = \frac{1}{3} \rho v_{rms}^2 \).
Q: What’s the difference between \(v_{rms}\), \(v_{avg}\), and \(v_{mp}\)?
A: \(v_{rms}\) is the root mean square (highest), \(v_{avg}\) is the average, and \(v_{mp}\) is the most probable velocity, all from the Maxwell-Boltzmann distribution.
Q: Can temperature or molar mass be negative?
A: No, both must be positive for physical meaning.
Q: Why does the result show zero?
A: If temperature or molar mass is zero, results default to zero.
Q: Why are some results in scientific notation?
A: Values < 0.0001 are displayed as, e.g., \(1.23 \times 10^{-5}\), for clarity.
Q: What does Hz mean here?
A: Hz is included as \( v_{rms} / 2\pi \), representing frequency (non-standard but per request), assuming a cyclical context.
Q: How do I use the gas dropdown?
A: Select a gas to auto-fill molar mass in g/mol, or enter a custom value in kg/mol or g/mol.