 Home
Home
 Back
Back
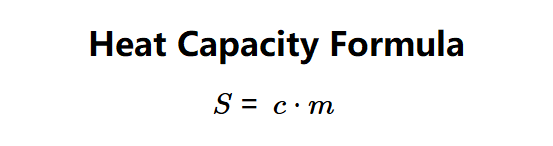
Definition: This calculator computes the heat capacity (\( S \)) of a substance, using the formula \( S = c \cdot m \), based on the mass and specific heat capacity of the substance.
Purpose: It is used in physics, chemistry, and engineering to determine how much heat energy a substance can absorb or release per unit temperature change, aiding in thermal analysis and system design.
The calculator uses the following formula:
Where:
Steps:
Calculating heat capacity is crucial for:
Example 1: Calculate \( S \) for water with the following properties:
Example 2 (Custom Specific Heat): Calculate \( S \) with a custom specific heat:
Q: What does heat capacity represent?
A: Heat capacity (\( S \)) is the amount of heat energy required to raise the temperature of a substance by 1 Kelvin (or 1°C), measured in units like J/K or J/°C.
Q: Why are K and °C interchangeable in this context?
A: Heat capacity relates to temperature differences, and a change of 1 K equals a change of 1°C, making the units interchangeable for this calculation (though absolute temperatures differ by 273.15).
Q: Can this calculator be used for any substance?
A: Yes, you can use the predefined list of substances or input a custom specific heat capacity for any substance, as long as the specific heat value is known.