1. What is an Arterial Blood pH Calculator?
Definition: The Arterial Blood pH Calculator estimates the pH of arterial blood using the Henderson-Hasselbalch equation, based on bicarbonate concentration and the partial pressure of CO₂.
Purpose: It helps clinicians evaluate acid-base balance in the body, aiding in the diagnosis of conditions like respiratory or metabolic acidosis/alkalosis.
2. How Does the Calculator Work?
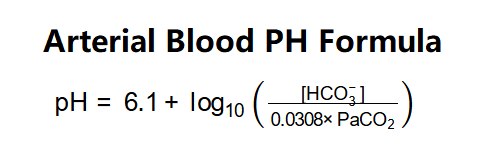
The calculator uses the following formula:
Unit Conversions (if needed):
- PaCO₂: 1 kPa = 7.50062 mmHg
Steps:
- Input the bicarbonate concentration (HCO₃⁻) in mmol/L and the partial pressure of CO₂ (PaCO₂), selecting the unit (mmHg or kPa).
- Validate inputs (both values must be greater than zero).
- Convert PaCO₂ to mmHg if provided in kPa.
- Calculate the denominator: \( 0.0308 \times \text{PaCO}_2 \).
- Compute the ratio: \( \frac{[\text{HCO}_3^-]}{0.0308 \times \text{PaCO}_2} \).
- Apply the Henderson-Hasselbalch equation: \( \text{pH} = 6.1 + \log_{10}(\text{ratio}) \).
- Display the pH result, rounded to 2 decimal places.
3. Importance of Arterial Blood pH Calculations
Calculating arterial blood pH is important for:
- Diagnosing Acid-Base Disorders: Identifies conditions like respiratory acidosis (low pH, high PaCO₂) or metabolic alkalosis (high pH, high HCO₃⁻).
- Guiding Treatment: Helps clinicians determine appropriate interventions, such as ventilation adjustments or bicarbonate administration.
- Monitoring Critical Patients: Essential in critical care settings to monitor patients with respiratory or metabolic disturbances.
4. Using the Calculator
Examples:
- Example 1: Bicarbonate: 24 mmol/L, PaCO₂: 40 mmHg
- Denominator: \( 0.0308 \times 40 = 1.232 \)
- Ratio: \( \frac{24}{1.232} = 19.48 \)
- pH: \( 6.1 + \log_{10}(19.48) = 6.1 + 1.290 = 7.39 \)
- Example 2: Bicarbonate: 15 mmol/L, PaCO₂: 6.67 kPa
- Convert PaCO₂: \( 6.67 \times 7.50062 = 50.03 \text{ mmHg} \)
- Denominator: \( 0.0308 \times 50.03 = 1.541 \)
- Ratio: \( \frac{15}{1.541} = 9.73 \)
- pH: \( 6.1 + \log_{10}(9.73) = 6.1 + 0.988 = 7.09 \)
5. Frequently Asked Questions (FAQ)
Q: What does a low arterial pH indicate?
A: A pH below 7.35 indicates acidosis, which may be due to respiratory issues (high PaCO₂) or metabolic causes (low HCO₃⁻).
Q: What is a normal arterial blood pH?
A: Normal arterial blood pH ranges from 7.35 to 7.45.
Q: How can I correct an abnormal pH?
A: Treatment depends on the cause—e.g., improving ventilation for respiratory acidosis or administering bicarbonate for metabolic acidosis—consult a healthcare provider.
 Home
Home
 Back
Back