1. What is the Water Heating Calculator?
Definition: This calculator computes the total energy (\( Q_{\text{total}} \)) required to heat a given mass or volume of water from an initial temperature to a final temperature, including phase changes if applicable (e.g., melting ice), and the time required based on heating power and efficiency.
Purpose: It is used in thermodynamics and engineering to estimate energy and time requirements for heating water, such as in household applications (e.g., brewing tea, heating water for cooking) or industrial processes (e.g., heating systems).
2. How Does the Calculator Work?
The calculator uses the following equations:
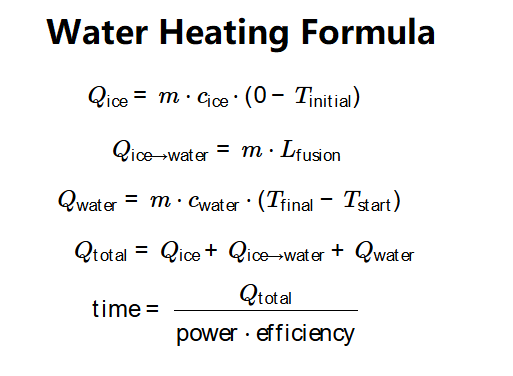
Formulas:
- Heat to raise ice to 0°C (if \( T_{\text{initial}} < 0^\circ \text{C} \)): \[
Q_{\text{ice}} = m \cdot c_{\text{ice}} \cdot (0 - T_{\text{initial}})
\]
- Heat to melt ice to water at 0°C: \[
Q_{\text{ice} \to \text{water}} = m \cdot L_{\text{fusion}}
\]
- Heat to raise water to final temperature: \[
Q_{\text{water}} = m \cdot c_{\text{water}} \cdot (T_{\text{final}} - T_{\text{start}})
\]
- Total Energy: \[
Q_{\text{total}} = Q_{\text{ice}} + Q_{\text{ice} \to \text{water}} + Q_{\text{water}}
\]
- Time: \[
\text{time} = \frac{Q_{\text{total}}}{\text{power} \cdot \text{efficiency}}
\]
where:
- \( m \): Mass of water in kg, g, lb (or derived from volume in L, mL, m³, ft³)
- \( c_{\text{ice}} \): Specific heat of ice, 2,108 J/(kg·K)
- \( c_{\text{water}} \): Specific heat of water, 4,190 J/(kg·K)
- \( L_{\text{fusion}} \): Latent heat of fusion, 334,000 J/kg
- \( T_{\text{initial}}, T_{\text{final}} \): Initial and final temperatures in K, °C, °F
- \( T_{\text{start}} \): Starting temperature for water phase (0°C if melting, otherwise \( T_{\text{initial}} \))
- \( Q_{\text{total}} \): Total energy in J, kJ (default), MJ, Wh, kWh, ft-lb, kcal
- \( \text{power} \): Heating power in W, kW
- \( \text{efficiency} \): Efficiency as a decimal (e.g., 90% = 0.9)
- \( \text{time} \): Time in s, min, hr
Unit Conversions:
- Mass (\( m \)):
- 1 kg = 1 kg
- 1 g = 0.001 kg
- 1 lb = 0.45359237 kg
- Volume (converted to mass, assuming 1 L = 1 kg):
- 1 L = 0.001 m³
- 1 mL = 0.000001 m³
- 1 m³ = 1 m³
- 1 ft³ = 0.0283168466 m³
- 1 m³ of water = 1000 kg (density of water = 1000 kg/m³)
- Temperature (\( T_{\text{initial}}, T_{\text{final}} \)):
- Kelvin (K): No conversion needed
- Celsius (°C) to Kelvin: \( T_K = T_C + 273.15 \)
- Fahrenheit (°F) to Kelvin: \( T_K = (T_F - 32) \cdot \frac{5}{9} + 273.15 \)
- Power:
- Energy (\( Q_{\text{total}} \)):
- 1 J = 1 J
- 1 kJ = 1000 J
- 1 MJ = \( 1 \times 10^6 \, \text{J} \)
- 1 Wh = 3600 J
- 1 kWh = \( 3.6 \times 10^6 \, \text{J} \)
- 1 ft-lb = \( \frac{1}{0.737562149I} \approx 1.35582 \, \text{J} \)
- 1 kcal = 4184 J
- Time:
- 1 s = 1 s
- 1 min = 60 s
- 1 hr = 3600 s
Steps:
- Select whether to input mass or volume of water.
- Enter the mass (in kg, g, lb) or volume (in L, mL, m³, ft³).
- Enter the initial and final temperatures in K, °C, or °F.
- Enter the heating power in W or kW.
- Enter the efficiency as a percentage (0-100%).
- Convert all inputs to SI units (kg for mass, Kelvin for temperature, W for power).
- Calculate the total energy \( Q_{\text{total}} \) by summing the heat required for each phase (ice to 0°C, melting, water heating).
- Calculate the time using the total energy, power, and efficiency.
- Convert the results to the selected units (defaulting to kJ for energy) and display them, using scientific notation if the absolute value is less than 0.001, otherwise rounded to 4 decimal places.
3. Importance of Water Heating Calculation
Calculating water heating requirements is crucial for:
- Household Applications: It helps estimate energy and time for tasks like brewing tea, cooking, or heating water for bathing.
- Energy Efficiency: Understanding energy needs aids in selecting efficient heating devices and optimizing energy consumption.
- Industrial Processes: It is essential for designing heating systems in industries like food processing, chemical manufacturing, and HVAC.
4. Using the Calculator
Examples:
- Example 1: Calculate the energy and time to heat 1 kg of ice at -10°C to water at 96°C using a 1800 W kettle with 90% efficiency:
- Select Input Type: Mass.
- Enter Mass = 1 kg.
- Enter Initial Temperature = -10°C, convert to K: \( -10 + 273.15 = 263.15 \, \text{K} \).
- Enter Final Temperature = 96°C, convert to K: \( 96 + 273.15 = 369.15 \, \text{K} \).
- Enter Heating Power = 1800 W.
- Enter Efficiency = 90% (\( 0.9 \)).
- Heat to raise ice to 0°C: \( Q_{\text{ice}} = 1 \times 2108 \times (273.15 - 263.15) = 21,080 \, \text{J} \).
- Heat to melt ice: \( Q_{\text{ice} \to \text{water}} = 1 \times 334000 = 334,000 \, \text{J} \).
- Heat to raise water to 96°C: \( Q_{\text{water}} = 1 \times 4190 \times (369.15 - 273.15) = 402,489 \, \text{J} \).
- Total Energy: \( Q_{\text{total}} = 21,080 + 334,000 + 402,489 = 757,569 \, \text{J} = 757.569 \, \text{kJ} \).
- Time: \( \text{time} = \frac{757,569}{1800 \times 0.9} = 467.636 \, \text{s} \approx 7.7939 \, \text{min} \).
- Results: \( Q_{\text{total}} = 757.569 \, \text{kJ} \), Time = 7.7939 min.
5. Frequently Asked Questions (FAQ)
Q: What does the calculator account for when the initial temperature is below 0°C?
A: If the initial temperature is below 0°C, the calculator accounts for heating the ice to 0°C, melting it to water at 0°C, and then heating the water to the final temperature.
Q: Why is efficiency important in the time calculation?
A: Efficiency reflects the actual power delivered to the water. A lower efficiency means more energy is lost (e.g., as heat to the surroundings), increasing the time required to heat the water.
Q: What are some real-world applications of this calculator?
A: It’s used for estimating energy and time for boiling water (e.g., for tea), designing water heaters, and optimizing industrial heating processes.
Water Heating Calculator© - All Rights Reserved 2025
 Home
Home
 Back
Back