1. What is Thermal Efficiency Calculator?
Definition: This calculator computes the thermal efficiency of a heat engine by taking the heat received (\( Q_{in} \)) and heat rejected (\( Q_{out} \)) as inputs, calculating the net work output (\( W_{net,out} \)) and thermal efficiency (\( \eta_{th} \)). Thermal efficiency measures how much of the heat input is converted into useful work.
Purpose: It is used in thermodynamics and engineering to evaluate the performance of heat engines, such as those in power plants, engines, and refrigeration systems.
2. How Does the Calculator Work?
The calculator uses the following formulas:
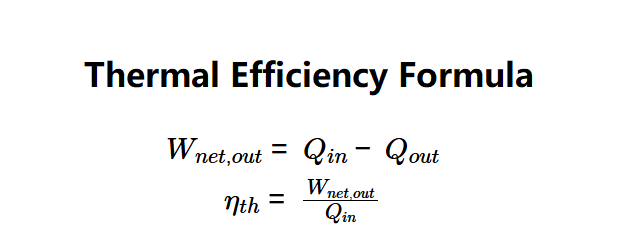
- \( W_{net,out} = Q_{in} - Q_{out} \)
- \( \eta_{th} = \frac{W_{net,out}}{Q_{in}} \)
Where:
- \( Q_{in} \): Heat received (e.g., J);
- \( Q_{out} \): Heat rejected (e.g., J);
- \( W_{net,out} \): Net work output (e.g., J);
- \( \eta_{th} \): Thermal efficiency (%).
Steps:
- Enter the heat received (\( Q_{in} \)) and heat rejected (\( Q_{out} \)) with their respective units (J, kJ, MJ, Wh, kWh, MWh, cal, kcal, BTU, MMBTU, thm, or BTU/lb).
- Select the desired unit for net work output (\( W_{net,out} \)).
- Convert inputs to Joules.
- Calculate the net work output and thermal efficiency using the formulas.
- Convert the net work output to the selected unit.
- Display the results, formatted in scientific notation if the absolute value is less than 0.001, otherwise with 4 decimal places.
3. Importance of Thermal Efficiency Calculation
Calculating thermal efficiency is crucial for:
- Engine Performance: Evaluating how efficiently a heat engine converts heat into work, which is essential for optimizing power generation and fuel usage.
- Energy Conservation: Identifying opportunities to reduce energy waste in thermal systems like engines and refrigerators.
- Thermodynamic Analysis: Understanding the efficiency of real engines to improve designs and performance.
4. Using the Calculator
Example 1: Calculate thermal efficiency and net work output:
- Heat Received: \( Q_{in} = 1000 \, \text{BTU/lb} \);
- Heat Rejected: \( Q_{out} = 600 \, \text{BTU/lb} \);
- Net Work Output Unit: BTU/lb;
- Convert to Joules: \( Q_{in} = 1000 \times 2326 = 2,326,000 \, \text{J} \), \( Q_{out} = 600 \times 2326 = 1,395,600 \, \text{J} \);
- Net Work Output: \( W_{net,out} = 2,326,000 - 1,395,600 = 930,400 \, \text{J} \);
- Convert back: \( W_{net,out} = 930,400 / 2326 = 400 \, \text{BTU/lb} \);
- Thermal Efficiency: \( \eta_{th} = \frac{930,400}{2,326,000} \times 100 \approx 40\% \);
- Result: \( Q_{in} = 1000.0000 \, \text{BTU/lb} \), \( Q_{out} = 600.0000 \, \text{BTU/lb} \), \( W_{net,out} = 400.0000 \, \text{BTU/lb} \), \( \eta_{th} = 40.0000\% \).
Example 2: Calculate with different units:
- Heat Received: \( Q_{in} = 5000 \, \text{kJ} \);
- Heat Rejected: \( Q_{out} = 3 \, \text{MWh} \);
- Net Work Output Unit: kWh;
- Convert to Joules: \( Q_{in} = 5000 \times 1000 = 5,000,000 \, \text{J} \), \( Q_{out} = 3 \times 3600000000 = 10,800,000,000 \, \text{J} \);
- Net Work Output: \( W_{net,out} = 5,000,000 - 10,800,000,000 = -10,795,000,000 \, \text{J} \);
- Since \( Q_{out} > Q_{in} \), this results in an error: "Heat rejected must be less than heat received."
Corrected Example 2: Adjust inputs to be physically valid:
- Heat Received: \( Q_{in} = 5 \, \text{MWh} \);
- Heat Rejected: \( Q_{out} = 3000 \, \text{kJ} \);
- Net Work Output Unit: kWh;
- Convert to Joules: \( Q_{in} = 5 \times 3600000000 = 18,000,000,000 \, \text{J} \), \( Q_{out} = 3000 \times 1000 = 3,000,000 \, \text{J} \);
- Net Work Output: \( W_{net,out} = 18,000,000,000 - 3,000,000 = 17,997,000,000 \, \text{J} \);
- Convert back: \( W_{net,out} = 17,997,000,000 / 3600000 = 4999.1667 \, \text{kWh} \);
- Thermal Efficiency: \( \eta_{th} = \frac{17,997,000,000}{18,000,000,000} \times 100 \approx 99.9833\% \);
- Result: \( Q_{in} = 5.0000 \, \text{MWh} \), \( Q_{out} = 3000.0000 \, \text{kJ} \), \( W_{net,out} = 4999.1667 \, \text{kWh} \), \( \eta_{th} = 99.9833\% \).
5. Frequently Asked Questions (FAQ)
Q: What is thermal efficiency?
A: Thermal efficiency (\( \eta_{th} \)) is the ratio of useful work output to the heat input in a heat engine, expressed as a percentage, indicating how efficiently heat is converted into work.
Q: Why must heat received be greater than heat rejected?
A: For a heat engine to produce positive work, the heat received (\( Q_{in} \)) must exceed the heat rejected (\( Q_{out} \)), as \( W_{net,out} = Q_{in} - Q_{out} \). If \( Q_{out} \geq Q_{in} \), no useful work is produced.
Q: What units can I use for heat values?
A: You can use Joules (J), kilojoules (kJ), megajoules (MJ), watt hours (Wh), kilowatt hours (kWh), megawatt hours (MWh), calories (cal), kilocalories (kcal), British thermal units (BTU), million BTU (MMBTU), therms (thm), or BTU per pound (BTU/lb). Each variable can have its own unit, and the calculator converts them to Joules for calculation.
Thermal Efficiency Calculator© - All Rights Reserved 2025
 Home
Home
 Back
Back