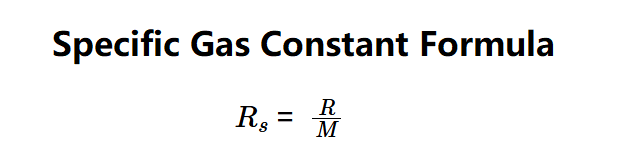1. What is Specific Gas Constant Calculator?
Definition: This calculator computes the specific gas constant (\( R_s \)) of a gas by dividing the universal gas constant (\( R \)) by the molar mass of the gas (\( M \)).
Purpose: It is used in thermodynamics and gas dynamics to characterize the behavior of a specific gas in the ideal gas law, aiding in calculations involving pressure, volume, and temperature.
2. How Does the Calculator Work?
The calculator uses the specific gas constant formula:
Where:
- \( R \): Universal gas constant (default: \( 8.314462618 \, \text{J·K}^{-1}\text{·mol}^{-1} \));
- \( M \): Molar mass of the gas (kg/mol);
- \( R_s \): Specific gas constant (J/(kg·K)).
Steps:
- Enter the universal gas constant (\( R \)) and its unit (J/(mol·K), cal/(mol·K), or erg/(mol·K)).
- Enter the molar mass (\( M \)) and its unit (kg/mol or g/mol).
- Convert the universal gas constant to J/(mol·K) and molar mass to kg/mol.
- Calculate the specific gas constant using the formula.
- Convert the specific gas constant to the selected output unit (J/(kg·K), kJ/(kg·K), or cal/(kg·K)).
- Display the result, formatted in scientific notation if the absolute value is less than 0.001, otherwise with 4 decimal places.
3. Importance of Specific Gas Constant Calculation
Calculating the specific gas constant is crucial for:
- Thermodynamics: Using the ideal gas law (\( PV = m R_s T \)) to relate pressure, volume, and temperature for a specific gas.
- Gas Dynamics: Analyzing the behavior of gases in processes like compression, expansion, and flow.
- Engineering Applications: Designing systems involving gases, such as engines, HVAC systems, and chemical reactors.
4. Using the Calculator
Example 1 (Default Universal Gas Constant): Calculate the specific gas constant for air:
- Universal Gas Constant: \( R = 8.314462618 \, \text{J/(mol·K)} \);
- Molar Mass: \( M = 0.02896 \, \text{kg/mol} \);
- Specific Gas Constant: \( R_s = \frac{8.314462618}{0.02896} \approx 287.0312 \, \text{J/(kg·K)} \);
- Result: \( R_s = 287.0312 \, \text{J/(kg·K)} \).
Example 2 (Custom Universal Gas Constant, Different Units): Calculate the specific gas constant with a custom universal gas constant:
- Universal Gas Constant: \( R = 1.987 \, \text{cal/(mol·K)} \);
- Molar Mass: \( M = 28.96 \, \text{g/mol} = 0.02896 \, \text{kg/mol} \);
- Convert \( R \): \( R = 1.987 \times 4.186 = 8.317582 \, \text{J/(mol·K)} \);
- Specific Gas Constant: \( R_s = \frac{8.317582}{0.02896} \approx 287.1417 \, \text{J/(kg·K)} \);
- Result in cal/(kg·K): \( R_s = 287.1417 / 4.186 \approx 68.5991 \, \text{cal/(kg·K)} \).
5. Frequently Asked Questions (FAQ)
Q: What is the specific gas constant?
A: The specific gas constant (\( R_s \)) is the universal gas constant (\( R \)) divided by the molar mass of a gas, making it specific to that gas and useful in the ideal gas law.
Q: Why is the molar mass in kg/mol?
A: The molar mass in kg/mol ensures that the specific gas constant has units of J/(kg·K), which is consistent with the ideal gas law when mass is in kg.
Q: Why might I need to change the universal gas constant?
A: You might change the universal gas constant if you're working with a different set of units or a more precise value for specific applications.
Specific Gas Constant Calculator© - All Rights Reserved 2025
 Home
Home
 Back
Back