1. What is Sensible Heat Calculator?
Definition: This calculator computes the sensible heat (\( Q \)) required to change the temperature of an object without changing its phase, based on its mass, specific heat capacity, and temperature difference.
Purpose: It is used in physics, engineering, and thermodynamics to determine the heat energy involved in processes like heating or cooling, aiding in the design of HVAC systems, thermal management, and material processing.
2. How Does the Calculator Work?
The calculator uses the sensible heat formula:
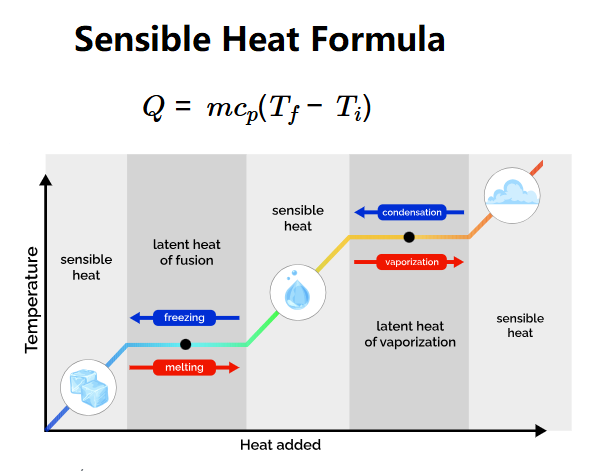
- \( Q = m c_p (T_f - T_i) \)
Where:
- \( Q \): Sensible heat (cal);
- \( m \): Mass of the object (g);
- \( c_p \): Specific heat capacity (cal/(g·K));
- \( T_f \): Final temperature (°C);
- \( T_i \): Initial temperature (°C).
Steps:
- Enter the mass of the object (\( m \)) and its unit (g, kg, or lb).
- Enter the initial temperature (\( T_i \)) and its unit (°C, K, or °F).
- Enter the final temperature (\( T_f \)) and its unit.
- Choose to select a material from the list or input a custom specific heat capacity (\( c_p \)).
- If selecting a material, choose from the predefined list with associated specific heat capacities.
- If entering a custom value, provide the specific heat capacity and its unit (cal/(g·K), J/(kg·K), or Btu/(lb·°F)).
- Convert mass to grams, temperatures to Celsius, and specific heat to cal/(g·K).
- Calculate the sensible heat using the formula.
- Convert the sensible heat to the selected output unit (cal, J, kJ, or Btu).
- Display the result, formatted in scientific notation if the absolute value is less than 0.001, otherwise with 4 decimal places.
3. Importance of Sensible Heat Calculation
Calculating sensible heat is crucial for:
- Thermal Management: Determining the heat required to change the temperature of materials in HVAC systems, electronics cooling, and industrial processes.
- Energy Efficiency: Optimizing heating and cooling processes to minimize energy consumption.
- Material Science: Analyzing the thermal behavior of materials during manufacturing or testing.
4. Using the Calculator
Example 1 (Heating Water): Calculate the sensible heat required to heat water:
- Mass: \( m = 1 \, \text{g} \);
- Initial Temperature: \( T_i = 20 \, \text{°C} \);
- Final Temperature: \( T_f = 100 \, \text{°C} \);
- Material: Water (\( c_p = 1.000 \, \text{cal/(g·K)} \));
- Sensible Heat: \( Q = 1 \times 1.000 \times (100 - 20) = 80 \, \text{cal} \);
- Result: \( Q = 80.0000 \, \text{cal} \).
Example 2 (Custom Specific Heat, Different Units): Calculate the sensible heat with a custom specific heat:
- Mass: \( m = 2 \, \text{lb} \);
- Initial Temperature: \( T_i = 68 \, \text{°F} \);
- Final Temperature: \( T_f = 212 \, \text{°F} \);
- Specific Heat: \( c_p = 0.2 \, \text{Btu/(lb·°F)} \approx 0.2 \, \text{cal/(g·K)} \);
- Convert units: \( m = 2 \times 453.592 = 907.184 \, \text{g} \), \( T_i = (68 - 32) \times 5/9 = 20 \, \text{°C} \), \( T_f = (212 - 32) \times 5/9 = 100 \, \text{°C} \);
- Sensible Heat: \( Q = 907.184 \times 0.2 \times (100 - 20) = 14514.944 \, \text{cal} \);
- Result in kJ: \( Q = 14514.944 \times 4.186 / 1000 = 60.7696 \, \text{kJ} \).
5. Frequently Asked Questions (FAQ)
Q: What is sensible heat?
A: Sensible heat (\( Q \)) is the heat energy required to change the temperature of an object without changing its phase, such as heating water from 20°C to 100°C.
Q: What does specific heat capacity represent?
A: Specific heat capacity (\( c_p \)) is the amount of heat required to raise the temperature of 1 gram of a material by 1 Kelvin (or 1°C), reflecting the material's ability to store thermal energy.
Q: Why does the temperature difference matter?
A: The temperature difference (\( T_f - T_i \)) determines the amount of heat absorbed or released; a larger difference requires more heat to achieve the temperature change.
Sensible Heat Calculator© - All Rights Reserved 2025
 Home
Home
 Back
Back