1. What is the Mixing Ratio of Air Calculator?
Definition: This calculator computes the actual mixing ratio (\( w \)), saturation mixing ratio (\( w_s \)), and relative humidity (\( \text{RH} \)) of air based on the air temperature, dew point temperature, and station pressure. The mixing ratio represents the mass of water vapor per unit mass of dry air.
Purpose: It is used in meteorology to analyze the moisture content of the air, which is critical for weather forecasting, understanding cloud formation, and assessing atmospheric stability.
2. How Does the Calculator Work?
The calculator uses the following formulas:
Formulas:
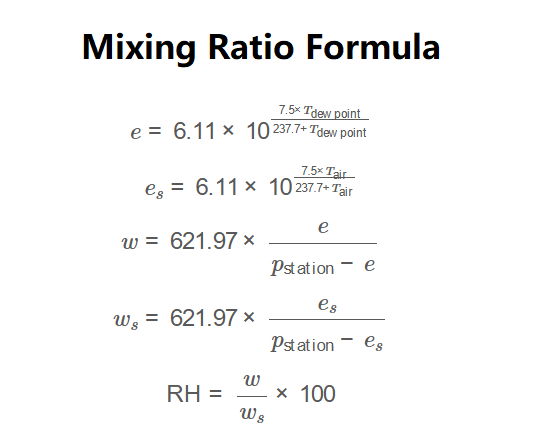
\[
e = 6.11 \times 10^{\frac{7.5 \times T_{\text{dew point}}}{237.7 + T_{\text{dew point}}}}
\]
\[
e_s = 6.11 \times 10^{\frac{7.5 \times T_{\text{air}}}{237.7 + T_{\text{air}}}}
\]
\[
w = 621.97 \times \frac{e}{p_{\text{station}} - e}
\]
\[
w_s = 621.97 \times \frac{e_s}{p_{\text{station}} - e_s}
\]
\[
\text{RH} = \frac{w}{w_s} \times 100
\]
where:
- \( e \): Actual vapor pressure (hPa)
- \( e_s \): Saturated vapor pressure (hPa)
- \( w \): Actual mixing ratio (g/kg, g/lb)
- \( w_s \): Saturation mixing ratio (g/kg, g/lb)
- \( \text{RH} \): Relative humidity (%)
- \( T_{\text{dew point}} \): Dew point temperature (°C, °F, K)
- \( T_{\text{air}} \): Air temperature (°C, °F, K)
- \( p_{\text{station}} \): Station pressure (hPa, Pa, bar, psi, at, atm, Torr, kPa, lb/ft², mmHg, inHg)
Unit Conversions:
- Temperature (Air Temperature, Dew Point):
- 1 °C = 1 °C
- 1 °F = (T - 32) × 5/9 °C
- 1 K = T - 273.15 °C
- Pressure (\( p_{\text{station}} \)):
- 1 hPa = 1 hPa
- 1 Pa = 0.01 hPa
- 1 bar = 1000 hPa
- 1 psi = 68.9476 hPa
- 1 at = 980.665 hPa
- 1 atm = 1013.25 hPa
- 1 Torr = 1.33322 hPa
- 1 kPa = 10 hPa
- 1 lb/ft² = 0.478803 hPa
- 1 mmHg = 1.33322 hPa
- 1 inHg = 33.8639 hPa
- Mixing Ratio (\( w \), \( w_s \)):
- 1 g/kg = 1 g/kg
- 1 g/lb = 1 g/kg × 0.453592
Steps:
- Enter the air temperature in °C, °F, or K (default is 25°C, step size 0.00001).
- Enter the dew point in °C, °F, or K (default is 15°C, step size 0.00001).
- Enter the station pressure in hPa, Pa, bar, psi, at, atm, Torr, kPa, lb/ft², mmHg, or inHg (default is 1013.25 hPa, step size 0.00001).
- Convert air temperature and dew point to Celsius (°C), and pressure to hPa.
- Calculate the actual vapor pressure (\( e \)) using the dew point.
- Calculate the saturated vapor pressure (\( e_s \)) using the air temperature.
- Calculate the actual mixing ratio (\( w \)) using \( e \) and station pressure.
- Calculate the saturation mixing ratio (\( w_s \)) using \( e_s \) and station pressure.
- Calculate the relative humidity (\( \text{RH} \)) as the ratio of \( w \) to \( w_s \), expressed as a percentage.
- Convert the mixing ratios to the selected units and display all results, using scientific notation if the absolute value is less than 0.001, otherwise rounded to 4 decimal places.
3. Importance of Mixing Ratio Calculation
Calculating the mixing ratio and relative humidity is crucial for:
- Meteorology: Determining the moisture content of the air, which affects cloud formation, precipitation, and storm development.
- Aviation: Assessing atmospheric conditions that impact aircraft performance, such as engine efficiency and lift, especially in humid conditions.
- HVAC Systems: Designing systems to control humidity for comfort and to prevent issues like mold growth.
4. Using the Calculator
Examples:
- Example 1: Calculate the actual mixing ratio, saturation mixing ratio, and relative humidity with an air temperature of 25°C, dew point of 15°C, and station pressure of 1013.25 hPa, with mixing ratios in g/kg:
- Enter Air Temperature = 25 °C.
- Enter Dew Point = 15 °C.
- Enter Station Pressure = 1013.25 hPa.
- Actual vapor pressure: \( e = 6.11 \times 10^{\frac{7.5 \times 15}{237.7 + 15}} = 6.11 \times 10^{0.445} = 17.0546 \, \text{hPa} \).
- Saturated vapor pressure: \( e_s = 6.11 \times 10^{\frac{7.5 \times 25}{237.7 + 25}} = 6.11 \times 10^{0.713} = 31.6829 \, \text{hPa} \).
- Actual mixing ratio: \( w = 621.97 \times \frac{17.0546}{1013.25 - 17.0546} = 621.97 \times \frac{17.0546}{996.1954} = 10.6462 \, \text{g/kg} \).
- Saturation mixing ratio: \( w_s = 621.97 \times \frac{31.6829}{1013.25 - 31.6829} = 621.97 \times \frac{31.6829}{981.5671} = 20.0795 \, \text{g/kg} \).
- Relative humidity: \( \text{RH} = \frac{10.6462}{20.0795} \times 100 = 53.0085\% \).
- Result: \( w = 10.6462 \, \text{g/kg} \), \( w_s = 20.0795 \, \text{g/kg} \), \( \text{RH} = 53.0085\% \).
- Example 2: Calculate the actual mixing ratio, saturation mixing ratio, and relative humidity with an air temperature of 86°F, dew point of 59°F, and station pressure of 29.92 inHg, with mixing ratios in g/lb:
- Enter Air Temperature = 86 °F, convert to °C: \( (86 - 32) \times 5/9 = 30 \, \text{°C} \).
- Enter Dew Point = 59 °F, convert to °C: \( (59 - 32) \times 5/9 = 15 \, \text{°C} \).
- Enter Station Pressure = 29.92 inHg, convert to hPa: \( 29.92 \times 33.8639 = 1013.21 \, \text{hPa} \).
- Actual vapor pressure: \( e = 6.11 \times 10^{\frac{7.5 \times 15}{237.7 + 15}} = 17.0546 \, \text{hPa} \).
- Saturated vapor pressure: \( e_s = 6.11 \times 10^{\frac{7.5 \times 30}{237.7 + 30}} = 6.11 \times 10^{0.840} = 42.466 \, \text{hPa} \).
- Actual mixing ratio: \( w = 621.97 \times \frac{17.0546}{1013.21 - 17.0546} = 621.97 \times \frac{17.0546}{996.1554} = 10.6482 \, \text{g/kg} \).
- Convert to g/lb: \( 10.6482 \times 0.453592 = 4.8295 \).
- Saturation mixing ratio: \( w_s = 621.97 \times \frac{42.466}{1013.21 - 42.466} = 621.97 \times \frac{42.466}{970.744} = 27.2198 \, \text{g/kg} \).
- Convert to g/lb: \( 27.2198 \times 0.453592 = 12.3468 \).
- Relative humidity: \( \text{RH} = \frac{10.6482}{27.2198} \times 100 = 39.1159\% \).
- Result: \( w = 4.8295 \, \text{g/lb} \), \( w_s = 12.3468 \, \text{g/lb} \), \( \text{RH} = 39.1159\% \).
5. Frequently Asked Questions (FAQ)
Q: What is the mixing ratio?
A: The mixing ratio is the mass of water vapor per unit mass of dry air, typically expressed in grams of water vapor per kilogram of dry air (g/kg). The actual mixing ratio (\( w \)) reflects the current moisture content, while the saturation mixing ratio (\( w_s \)) indicates the maximum moisture the air can hold at a given temperature and pressure.
Q: How does relative humidity relate to mixing ratio?
A: Relative humidity is the ratio of the actual mixing ratio to the saturation mixing ratio, expressed as a percentage. It indicates how close the air is to saturation; a higher relative humidity means the air is closer to holding the maximum amount of water vapor possible at that temperature.
Q: Why is station pressure important?
A: Station pressure (\( p_{\text{station}} \)) is used to calculate the mixing ratios because it represents the total atmospheric pressure at the location, which affects the air's capacity to hold water vapor. It must be greater than the vapor pressures (\( e \) and \( e_s \)) to avoid invalid calculations.
Mixing Ratio of Air Calculator© - All Rights Reserved 2025
 Home
Home
 Back
Back