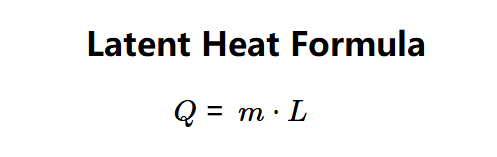1. What is Latent Heat Calculator?
Definition: This calculator computes the energy (\( Q \)) released or absorbed during a phase transition of a substance, such as melting, vaporization, or sublimation, using the latent heat formula.
Purpose: It is used in physics and chemistry to determine the energy required for phase changes, which is crucial for applications like material processing, refrigeration, and climate studies.
2. How Does the Calculator Work?
The calculator uses the latent heat formula:
Where:
- \( Q \): Latent heat (J);
- \( m \): Mass of the substance (kg);
- \( L \): Specific latent heat of the substance (J/kg).
Steps:
- Enter the mass of the substance (\( m \)) and its unit (kg, g, or lb).
- Choose the method to input the specific latent heat: select a predefined substance or enter a custom value.
- If selecting a substance, choose from the list which includes the phase transition (e.g., fusion or vaporization) and its specific latent heat.
- If entering a custom value, provide the specific latent heat (\( L \)) and its unit (J/kg, kJ/kg, or cal/g).
- Convert all inputs to base units (kg for mass, J/kg for specific latent heat).
- Calculate the latent heat \( Q \) using the formula.
- Convert the result to the selected output unit (J, kJ, MJ, Wh, kWh, cal, or kcal).
- Display the result, formatted in scientific notation if the absolute value is less than 0.001, otherwise with 4 decimal places.
3. Importance of Latent Heat Calculation
Calculating latent heat is crucial for:
- Phase Transition Analysis: Understanding the energy required for processes like melting ice or boiling water in industrial applications.
- Climate Studies: Analyzing the role of latent heat in weather phenomena, such as the energy released during condensation in the atmosphere.
- Engineering Design: Designing systems like refrigerators, heat pumps, and HVAC systems that rely on phase changes for efficient operation.
4. Using the Calculator
Example 1 (Melting Ice): Calculate the latent heat required to melt ice:
- Mass: \( m = 5 \, \text{kg} \);
- Substance: Water (Fusion/Melting, \( L = 334 \, \text{kJ/kg} \));
- Latent Heat: \( Q = 5 \times 334000 = 1670000 \, \text{J} \);
- Result in kJ: \( Q = 1670000 / 1000 = 1670.0000 \, \text{kJ} \);
- Result in kcal: \( Q = 1670000 / 4184 \approx 399.1392 \, \text{kcal} \).
Example 2 (Boiling Nitrogen): Calculate the latent heat required to vaporize nitrogen:
- Mass: \( m = 200 \, \text{g} \);
- Substance: Nitrogen (Vaporization/Boiling, \( L = 199 \, \text{kJ/kg} \));
- Convert units: \( m = 200 / 1000 = 0.2 \, \text{kg} \);
- Latent Heat: \( Q = 0.2 \times 199000 = 39800 \, \text{J} \);
- Result in kJ: \( Q = 39800 / 1000 = 39.8000 \, \text{kJ} \);
- Result in Wh: \( Q = 39800 / 3600 \approx 11.0556 \, \text{Wh} \).
5. Frequently Asked Questions (FAQ)
Q: What is latent heat?
A: Latent heat is the energy absorbed or released by a substance during a phase transition (e.g., melting, vaporization) without a change in temperature.
Q: How does latent heat differ from sensible heat?
A: Latent heat causes a phase change without changing the temperature, while sensible heat causes a temperature change without a phase change.
Q: Why does vaporization require more energy than fusion?
A: Vaporization involves breaking stronger intermolecular forces to transition from liquid to gas, requiring more energy than the solid-to-liquid transition during fusion.
 Home
Home
 Back
Back