 Home
Home
 Back
Back
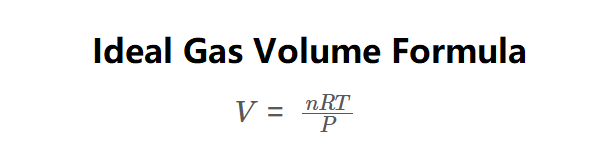
Definition: This calculator computes the volume (\( V \)) of an ideal gas based on the number of moles (\( n \)), temperature (\( T \)), and pressure (\( P \)).
Purpose: It is used in physics and chemistry to determine the volume occupied by a gas under ideal conditions, aiding in thermodynamic analysis and gas law applications.
The calculator uses the ideal gas law rearranged for volume:
\( V = \frac{nRT}{P} \)
Where:
Steps:
Calculating the volume of an ideal gas is crucial for:
Example 1: Calculate the volume with \( n = 1 \, \text{mol} \), \( T = 273.15 \, \text{K} \), \( P = 101325 \, \text{Pa} \):
Example 2: Calculate the volume with \( n = 0.5 \, \text{mol} \), \( T = 25 \, \text{°C} \), \( P = 1 \, \text{bar} \):
Q: What is the ideal gas law?
A: The ideal gas law (\( PV = nRT \)) relates pressure, volume, number of moles, and temperature for an ideal gas, assuming no intermolecular forces.
Q: Why must temperature be in Kelvin?
A: The ideal gas law requires absolute temperature in Kelvin for accurate calculations.
Q: Does this calculator apply to real gases?
A: This calculator uses the ideal gas law, which is an approximation. Real gases may deviate under high pressure or low temperature.