 Home
Home
 Back
Back
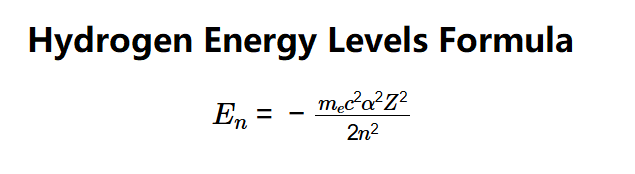
Definition: This calculator computes the energy (\( E_n \)) of an electron in a hydrogen-like atom at a specific energy level (\( n \)) for a given atomic number (\( Z \)).
Purpose: It is used in atomic physics to determine the quantized energy levels of electrons in hydrogen-like atoms, which is fundamental for understanding atomic spectra and quantum mechanics.
The calculator uses the following equation:
Where:
Steps:
Calculating the energy levels of hydrogen-like atoms is crucial for:
Example 1: Calculate the energy of an electron in a hydrogen atom (\( Z = 1 \)) at energy level \( n = 1 \):
Example 2 (Different \( Z \) and \( n \)): Calculate the energy for a helium ion (\( Z = 2 \)) at energy level \( n = 2 \):
Q: What does a negative energy value mean?
A: The negative energy indicates that the electron is in a bound state, with energy measured relative to the ionized state (\( E = 0 \)) where the electron is free.
Q: Why does the energy depend on \( Z^2 \)?
A: The energy scales with \( Z^2 \) because the Coulomb attraction between the electron and nucleus increases with the nuclear charge (\( Z \)), making the energy levels more negative (more tightly bound) for higher \( Z \).
Q: Can this calculator be used for multi-electron atoms?
A: This calculator is designed for hydrogen-like atoms (one electron). For multi-electron atoms, electron-electron interactions complicate the energy levels, requiring more advanced models.