 Home
Home
 Back
Back
Definition: This calculator computes the change in enthalpy (\( \Delta H \)) for a chemical reaction using the standard enthalpies of formation of the reactants and products.
Purpose: It is used in chemistry and thermodynamics to determine the heat absorbed or released during a reaction, aiding in the design of chemical processes and understanding reaction energetics.
The calculator uses the following formula:
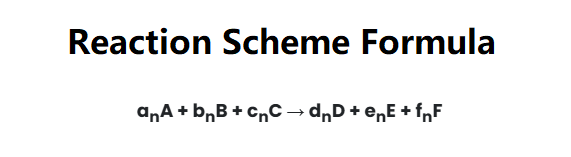
For the reaction \( aA + bB + cC \rightarrow dD + eE + fF \):
Where:
Steps:
Calculating the change in enthalpy is crucial for:
Example 1: Calculate \( \Delta H \) for the combustion of methane: \( \text{CH}_4(g) + 2\text{O}_2(g) \rightarrow \text{CO}_2(g) + 2\text{H}_2\text{O}(l) \)
Example 2: Calculate \( \Delta H \) for the formation of ammonia: \( \text{N}_2(g) + 3\text{H}_2(g) \rightarrow 2\text{NH}_3(g) \)
Q: What does a negative \( \Delta H \) indicate?
A: A negative \( \Delta H \) means the reaction is exothermic, releasing heat to the surroundings.
Q: Why are some \( \Delta H^\circ_f \) values zero?
A: Elements in their standard states (e.g., \( \text{O}_2(g) \), \( \text{H}_2(g) \)) have \( \Delta H^\circ_f = 0 \) by definition, as they are the reference points for enthalpy calculations.
Q: Can this calculator handle reactions with fewer substances?
A: Yes, set the coefficients of unused substances to 0 and select "None" for those substances.