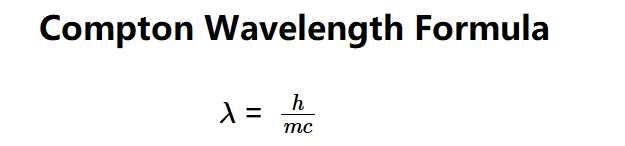1. What is the Compton Wavelength Calculator?
Definition: This calculator uses the Compton wavelength equation to compute the wavelength (\( \lambda \)) associated with a particle of mass \( m \).
Purpose: It is used in quantum physics to determine the wavelength of a photon with energy equal to the rest energy of a particle, which is fundamental in understanding Compton scattering and quantum mechanics.
2. How Does the Calculator Work?
The calculator uses the following equation:
- \( \lambda = \frac{h}{m c} \)
Where:
- \( \lambda \): Compton wavelength (m, cm, mm, nm, pm, or Å);
- \( h \): Planck's constant (\( 6.62607 \times 10^{-34} \, \text{J·s} \));
- \( m \): Mass of the particle (various units);
- \( c \): Speed of light (\( 299792458 \, \text{m/s} \)).
Steps:
- Enter the mass of the particle (\( m \)) with its unit.
- Convert the mass to kilograms.
- Calculate the Compton wavelength: \( \lambda = \frac{h}{m c} \).
- Convert the wavelength to the selected output unit and display \( \lambda \), formatted in scientific notation if the absolute value is less than 0.001, otherwise with 4 decimal places.
3. Importance of Compton Wavelength Calculation
Calculating the Compton wavelength is crucial for:
- Quantum Mechanics: Understanding the wave-particle duality of matter.
- Compton Scattering: Determining the wavelength shift in photon scattering by particles.
- Particle Physics: Studying the properties of fundamental particles like electrons and protons.
4. Using the Calculator
Example 1: Calculate \( \lambda \) for an electron:
- Mass: \( m = 1 \, m_e = 9.1093837 \times 10^{-31} \, \text{kg} \);
- Compton wavelength: \( \lambda = \frac{6.62607 \times 10^{-34}}{9.1093837 \times 10^{-31} \times 299792458} \approx 2.4263 \times 10^{-12} \, \text{m} \);
- Result: \( \lambda = 2.4263 \times 10^{-12} \, \text{m} \) or \( 2.4263 \, \text{nm} \).
Example 2 (Different Mass): Calculate \( \lambda \) for a proton:
- Mass: \( m = 1 \, m_p = 1.6726219 \times 10^{-27} \, \text{kg} \);
- Compton wavelength: \( \lambda = \frac{6.62607 \times 10^{-34}}{1.6726219 \times 10^{-27} \times 299792458} \approx 1.3214 \times 10^{-15} \, \text{m} \);
- Result: \( \lambda = 1.3214 \times 10^{-15} \, \text{m} \) or \( 1.3214 \, \text{pm} \).
5. Frequently Asked Questions (FAQ)
Q: What does the Compton wavelength represent?
A: It represents the wavelength of a photon whose energy equals the rest energy of the particle (\( E = m c^2 \)).
Q: Why does a larger mass result in a smaller wavelength?
A: The Compton wavelength is inversely proportional to the mass (\( \lambda \propto \frac{1}{m} \)), so a larger mass leads to a smaller wavelength.
Q: Is the Compton wavelength relevant for macroscopic objects?
A: For macroscopic objects (e.g., 1 kg), the Compton wavelength is extremely small (e.g., \( 2.2102 \times 10^{-42} \, \text{m} \)), making it negligible in classical physics.
Compton Wavelength Calculator© - All Rights Reserved 2025
 Home
Home
 Back
Back