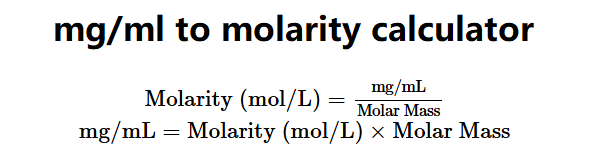mg/mL to Molarity and Molarity to mg/mL Calculator
How to Calculate Between mg/mL and Molarity
The mg/mL to Molarity and Molarity to mg/mL Calculator converts between milligrams per milliliter (mg/mL) and molarity (mol/L) for substances dissolved in water. The formulas are:
\( \text{Molarity (mol/L)} = \frac{\text{mg/mL}}{\text{Molar Mass}} \)
\( \text{mg/mL} = \text{Molarity (mol/L)} \times \text{Molar Mass} \)
Where:
- \( \text{mg/mL} \): Milligrams per milliliter, equivalent to g/L in water solutions.
- \( \text{Molarity (mol/L)} \): Moles of solute per liter of solution.
- \( \text{Molar Mass} \): Molecular or atomic weight in g/mol (e.g., 74.55 for KCl, 58.44 for NaCl).
Select the conversion direction, enter the input value and molar mass, then calculate the result.
Note: The conversion assumes 1 mg/mL = 1 g/L for water solutions, where 1 liter weighs approximately 1 kg.
Using the mg/mL and Molarity Mutual Conversion Calculator
This calculator is useful for chemical and environmental analysis, such as determining solute concentrations in water for laboratory or industrial applications.
Select the direction (mg/mL to Molarity or Molarity to mg/mL), input the value and molar mass, and the calculator outputs the converted value.
Example 1: For 10 mg/mL of KCl (molar mass 74.55 g/mol) to Molarity.
- Direction: mg/mL to Molarity
- Input: \( 10 \, \text{mg/mL} \)
- Molar Mass: \( 74.55 \, \text{g/mol} \)
- Calculation: \( \text{Molarity} = \frac{10}{74.55} = 0.134048 \, \text{mol/L} \)
- Result: \( 0.134048 \, \text{mol/L} \)
Example 2: For 0.5 mol/L of NaCl (molar mass 58.44 g/mol) to mg/mL.
- Direction: Molarity to mg/mL
- Input: \( 0.5 \, \text{mol/L} \)
- Molar Mass: \( 58.44 \, \text{g/mol} \)
- Calculation: \( \text{mg/mL} = 0.5 \times 58.44 = 29.22 \)
- Result: \( 29.22 \, \text{mg/mL} \)
Use this tool for precise conversions in chemical or water quality analysis.
Common FAQ
Below are frequently asked questions about mg/mL and Molarity conversions:
- Q: What is mg/mL?
A: mg/mL stands for milligrams per milliliter, equivalent to g/L, measuring mass concentration in water.
- Q: What is Molarity?
A: Molarity (mol/L) measures the number of moles of solute per liter of solution.
- Q: Why is molar mass needed?
A: Molar mass converts between mass-based (mg/mL) and mole-based (molarity) units.
- Q: How accurate is this calculator?
A: It provides precise results based on the formulas, with molarity rounded to 6 decimal places and mg/mL to 3 decimal places.
- Q: Can negative values be used?
A: No, input values and molar mass must be positive; invalid inputs leave the result blank.
 Home
Home
 Back
Back