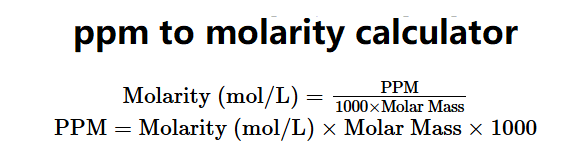PPM to Molarity and Molarity to PPM Calculator
How to Calculate Between PPM and Molarity
The PPM to Molarity and Molarity to PPM Calculator converts between parts per million (PPM) and molarity (mol/L) for substances dissolved in water. The formulas are:
\( \text{Molarity (mol/L)} = \frac{\text{PPM}}{1000 \times \text{Molar Mass}} \)
\( \text{PPM} = \text{Molarity (mol/L)} \times \text{Molar Mass} \times 1000 \)
Where:
- \( PPM \): Parts per million, equivalent to mg/L in water solutions.
- \( \text{Molarity (mol/L)} \): Moles of solute per liter of solution.
- \( \text{Molar Mass} \): Molecular or atomic weight in g/mol (e.g., 98.08 for H₂SO₄, 40 for NaOH).
Select the conversion direction, enter the input value and molar mass, then calculate the result.
Note: The conversion assumes 1 PPM = 1 mg/L for water solutions, where 1 liter weighs approximately 1 kg.
Using the PPM and Molarity Mutual Conversion Calculator
This calculator is useful for chemical and environmental analysis, such as determining solute concentrations in water for laboratory or industrial applications.
Select the direction (PPM to Molarity or Molarity to PPM), input the value and molar mass, and the calculator outputs the converted value.
Example 1: For 100 PPM of H₂SO₄ (molar mass 98.08 g/mol) to Molarity.
- Direction: PPM to Molarity
- Input: \( 100 \, \text{PPM} \)
- Molar Mass: \( 98.08 \, \text{g/mol} \)
- Calculation: \( \text{Molarity} = \frac{100}{1000 \times 98.08} = 0.001020 \, \text{mol/L} \)
- Result: \( 0.001020 \, \text{mol/L} \)
Example 2: For 0.0125 mol/L of NaOH (molar mass 40 g/mol) to PPM.
- Direction: Molarity to PPM
- Input: \( 0.0125 \, \text{mol/L} \)
- Molar Mass: \( 40 \, \text{g/mol} \)
- Calculation: \( \text{PPM} = 0.0125 \times 40 \times 1000 = 500 \)
- Result: \( 500 \, \text{PPM} \)
Use this tool for precise conversions in chemical or water quality analysis.
Common FAQ
Below are frequently asked questions about PPM and Molarity conversions:
- Q: What is PPM?
A: PPM stands for parts per million, equivalent to mg/L in water, measuring concentration.
- Q: What is Molarity?
A: Molarity (mol/L) measures the number of moles of solute per liter of solution.
- Q: Why is molar mass needed?
A: Molar mass converts between mass-based (PPM) and mole-based (molarity) units.
- Q: How accurate is this calculator?
A: It provides precise results based on the formulas, with molarity rounded to 6 decimal places and PPM to 3 decimal places.
- Q: Can negative values be used?
A: No, input values and molar mass must be positive; invalid inputs leave the result blank.
 Home
Home
 Back
Back