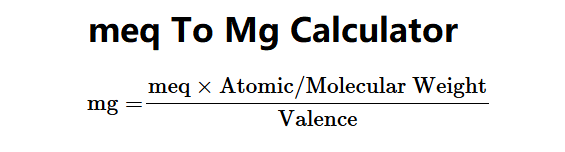Meq to Mg Calculator
How to Convert Meq to Mg
The Meq to Mg Converter calculates the weight in milligrams (mg) from milliequivalents (meq), atomic/molecular weight, and valence using the following formula:
\[ \text{mg} = \frac{\text{meq} \times \text{Atomic/Molecular Weight}}{\text{Valence}} \]
Where:
- \( \text{meq} \): Milliequivalents, a measure of chemical equivalents.
- \( \text{Atomic/Molecular Weight} \): The weight of one mole of the substance (converted to mg).
- \( \text{Valence} \): The number of equivalents per mole (unitless, typically an integer like 1 or 2).
- \( \text{mg} \): Milligrams, the resulting weight.
Select the unit for atomic/molecular weight (g, kg, mg), then enter the meq value, weight, and valence to calculate mg.
Using the Meq to Mg Converter
This converter is useful in chemistry for converting milliequivalents to milligrams based on atomic or molecular weight.
Input the milliequivalents (meq), atomic/molecular weight with its unit (g, kg, mg), and valence. The calculator converts the weight to milligrams and computes the result.
Example: Convert 2 meq of calcium (Ca²⁺) to mg, with an atomic weight of 0.04008 kg and valence of 2.
- Milliequivalents: \( 2 \, \text{meq} \)
- Atomic Weight: \( 0.04008 \, \text{kg} = 40,080 \, \text{mg} \)
- Valence: \( 2 \)
- mg: \( \frac{2 \times 40,080}{2} = 40,080 \, \text{mg} \)
- Result: \( 2 \, \text{meq} = 40,080 \, \text{mg} \)
Use this tool for accurate conversions in chemical calculations.
Common Conversion Table
Reference conversions for common ions (atomic weight in g, result in mg):
| Ion |
meq |
Atomic Weight (g) |
Valence |
mg |
| Na⁺ |
1 |
22.99 |
1 |
22990.000 |
| Ca²⁺ |
2 |
40.08 |
2 |
40080.000 |
| Mg²⁺ |
1 |
24.31 |
2 |
12155.000 |
| Cl⁻ |
5 |
35.45 |
1 |
177250.000 |
| HCO₃⁻ |
3 |
61.01 |
1 |
183.030000 |
Adjust atomic weight units using the form dropdown as needed.
Common FAQ
Below are frequently asked questions about Meq to Mg conversions:
- Q: What units can I use for atomic/molecular weight?
A: g, kg, or mg. The calculator converts all to milligrams.
- Q: Why is valence needed?
A: Valence determines the number of equivalents per mole, affecting the conversion.
- Q: Can negative values be used?
A: No, negative meq or atomic weight values are not meaningful. Valence must be positive.
- Q: How accurate is this converter?
A: Results are rounded to 6 decimal places using the exact formula.
 Home
Home
 Back
Back