 Home
Home
 Back
Back
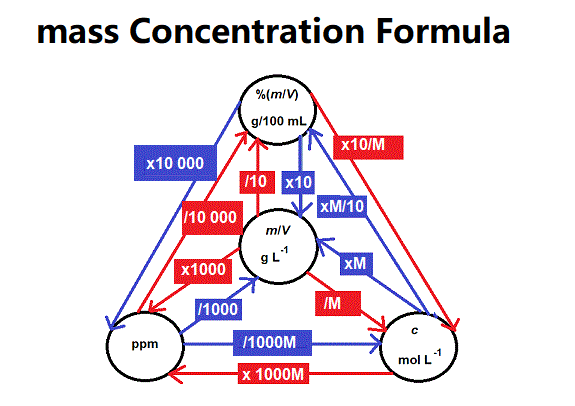
Definition: This converter transforms mass concentration values between various units. Mass concentration measures the mass of a solute per unit volume of solution, typically in grams per liter (g/L).
Purpose: Useful in chemistry, environmental science, and water quality analysis for converting concentration units in applications like pollution monitoring, solution preparation, and analytical chemistry.
The converter uses conversion factors relative to the base unit kilogram/liter [kg/L]:
Steps:
Mass concentration conversion is critical for:
Example 1: Convert 1 gram/liter to kilogram/liter:
Result: 0.00100 kilogram/liter
Example 2: Convert 1 milligram/liter to part/million (ppm):
Result: 1.00114 part/million (ppm)
Q: What is mass concentration?
A: Mass concentration is the mass of a solute dissolved in a given volume of solution, typically measured in grams per liter (g/L).
Q: Why are there different units for mass concentration?
A: Different fields use units like ppm for trace amounts, or imperial units like pound/gallon in specific regions or industries.
Q: How are g/L and mg/L related?
A: One g/L is equal to 1000 mg/L.
Q: Can this converter be used for all mass concentration scenarios?
A: Yes, it converts units of mass concentration, applicable to any scenario involving solute mass per solution volume.