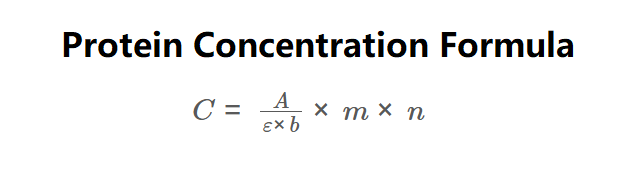1. What is the Protein Concentration Calculator?
Definition: This calculator computes the concentration of a protein sample based on its absorbance at a specific wavelength (\( \lambda_{\text{max}} \)), using the Beer-Lambert Law modified for protein properties.
Purpose: It is used in biochemistry to determine protein concentration in samples, which is essential for experiments like enzyme assays, protein purification, and structural studies.
2. How Does the Calculator Work?
The calculator uses the following formula:
\( C = \frac{A}{\varepsilon \times b} \times m \times n \)
Where:
- \( C \): Protein concentration (mg/mL, µg/mL);
- \( A \): Absorbance at \( \lambda_{\text{max}} \);
- \( \varepsilon \): Extinction coefficient (M⁻¹ cm⁻¹);
- \( b \): Pathlength of the cuvette (cm);
- \( m \): Molecular mass (g/mol);
- \( n \): Dilution factor.
Steps:
- Select a substance or choose "Custom" to input your own molecular mass and extinction coefficient.
- Enter the absorbance at \( \lambda_{\text{max}} \).
- Enter the pathlength of the cuvette (typically 1 cm).
- Enter the dilution factor (set to 1 if undiluted).
- Calculate the concentration using the formula.
- Convert the result to the selected output unit and display, formatted in scientific notation if the absolute value is less than 0.001, otherwise with 4 decimal places.
3. Importance of Protein Concentration Calculation
Calculating protein concentration is crucial for:
- Biochemical Assays: Ensures the correct protein amount for reactions like enzyme kinetics or antibody binding studies.
- Protein Purification: Monitors the yield and purity of protein samples during purification processes.
- Structural Studies: Provides accurate concentrations for techniques like X-ray crystallography or NMR spectroscopy.
4. Using the Calculator
Example 1: Calculate the concentration of BSA with an absorbance of 0.5, pathlength of 1 cm, and no dilution:
- Substance: BSA (Molecular Mass = 66,463 g/mol, Extinction Coefficient = 43,824 M⁻¹ cm⁻¹);
- Absorbance: \( A = 0.5 \);
- Pathlength: \( b = 1 \, \text{cm} \);
- Dilution Factor: \( n = 1 \);
- Concentration: \( C = \frac{0.5}{43,824 \times 1} \times 66,463 \times 1 \approx 0.758 \, \text{mg/mL} \);
- Result: \( C = 0.7580 \, \text{mg/mL} \).
Example 2: Calculate the concentration of a custom protein with an absorbance of 1.2, pathlength of 1 cm, dilution factor of 2, molecular mass of 50,000 g/mol, and extinction coefficient of 30,000 M⁻¹ cm⁻¹, outputting in µg/mL:
- Substance: Custom;
- Molecular Mass: \( m = 50,000 \, \text{g/mol} \);
- Extinction Coefficient: \( \varepsilon = 30,000 \, \text{M⁻¹ cm⁻¹} \);
- Absorbance: \( A = 1.2 \);
- Pathlength: \( b = 1 \, \text{cm} \);
- Dilution Factor: \( n = 2 \);
- Concentration: \( C = \frac{1.2}{30,000 \times 1} \times 50,000 \times 2 = 4 \, \text{mg/mL} = 4,000 \, \text{µg/mL} \);
- Result: \( C = 4000.0000 \, \text{µg/mL} \).
5. Frequently Asked Questions (FAQ)
Q: Why do I need the extinction coefficient?
A: The extinction coefficient (\( \varepsilon \)) quantifies how strongly a protein absorbs light at a specific wavelength, which is necessary to relate absorbance to concentration via the Beer-Lambert Law.
Q: What if I don’t know the extinction coefficient?
A: You can estimate it using the protein’s amino acid sequence (e.g., based on tryptophan, tyrosine, and cysteine content) or use a standard value for similar proteins.
Q: Can this calculator be used for other biomolecules?
A: Yes, if you know the molecular mass and extinction coefficient, the formula can be applied to other biomolecules that absorb light at a measurable wavelength.
Protein Concentration Calculator© - All Rights Reserved 2025
 Home
Home
 Back
Back